On Wednesday December 5, 2018, UACT, along with KEI, Social Security Works, Health GAP, Public Citizen, Dr. Ophira Ginsburg, and James Love, submitted joint comments in response to a proposed exclusive license for patents on CAR T technology for the treatment of cancers.
The Federal Register notice (83 FR 58262) “Prospective Grant of an Exclusive Patent License: Development and Commercialization of Chimeric Antigen Receptor (CAR) Therapies for the Treatment of FMS-Like Tyrosine Kinase 3 (FLT3) Expressing Cancers,” outlined the National Institutes of Health’s intent to grant an exclusive license on these technologies to ElevateBio, a company whose leadership includes the investors behind recent budget-breaking treatments, Sovaldi and Soliris. The full comments submitted follow below, and a PDF version is available here.
November 4, 2018
Jim Knabb
Senior Technology Transfer Manager
NCI Technology Transfer Center
Email: jim.knabb@nih.gov
Re: Prospective Grant of an Exclusive Patent License to ElevateBio
Dear Jim Knabb,
The following are comments by Knowledge Ecology International (KEI), the Union for Affordable Cancer Treatment (UACT), Social Security Works (SSW), Health GAP, Public Citizen, Ophira Ginsburg, and James Love regarding the “Prospective Grant of an Exclusive Patent License: Development and Commercialization of Chimeric Antigen Receptor (CAR) Therapies for the Treatment of FMS-Like Tyrosine Kinase 3 (FLT3) Expressing Cancers,” to ElevateBio, as noticed in the Federal Register notice 83 FR 58262.
Inventions to be licensed
E-133-2016: FLT3-Specific Chimeric Antigen Receptors and Methods Using Same
- US Provisional Patent Application 62/342,394, filed May 27, 2016 (E-133-2016-0-US-01);
- International Patent Application PCT/US2017/034,691, filed May 26, 2017 (E-133-2016-0-PCT-02)
“The development of a mono- or multi-specific FMS-like tyrosine kinase 3 (FLT3; also known as CD135) chimeric antigen receptor (CAR)-based immunotherapy using autologous or allogenic human lymphocytes (T cells or NK cells) transduced with lentiviral vectors, wherein the viral transduction leads to the expression of a CAR that targets FLT3 (comprised of the FLT3-binding domain referenced as NC7 in the invention as well as an intracellular signaling domain), for the prophylaxis or treatment of FLT3-expressing cancers.”
“This technology discloses a CAR vector that targets FLT3 comprised of an anti-FLT3 antibody known as NC7, and an intracellular signaling domain. FLT3 (CD135) is a cytokine receptor expressed on hematopoietic progenitor cells, and is one of the most frequently mutated genes in acute myeloid leukemia (AML) and infant acute lymphoblastic leukemia (ALL). FLT3 mutation leads to increased cell surface expression and therefore on leukemic cells, which makes it an attractive candidate for cellular therapies such as CAR-T.”
Global Incidence of Leukemia
According to the US Centers for Disease Control and Prevention (CDC), “Acute lymphoblastic leukemia (ALL) is the most prevalent cancer among children and adolescents in the United States, representing 20% of all cancers diagnosed in persons aged <20 years.”
Globally, the U.S. has a relatively high rate of incidence of leukemia in general, and AML and ALL in particular, but the bulk of the cases are outside the United States.
The following data are a table and two figures from: “Epidemiological patterns of leukaemia in 184 countries: a population-based study.” The Lancet Haematology. Vol. 5, Issue 1. January 1, 2018. DOI: https://doi.org/10.1016/S2352-3026(17)30232-6
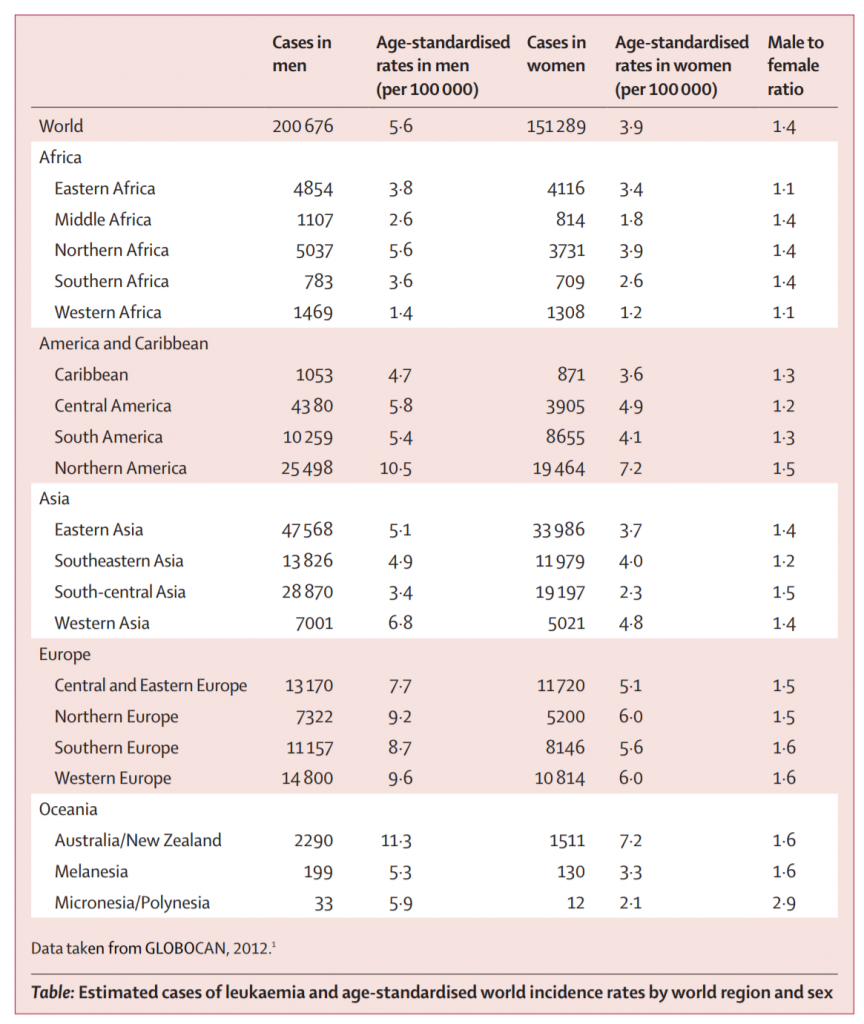
Note that North America collectively accounts for less than 13 percent of cases. Nearly half the cases worldwide are in Asia.
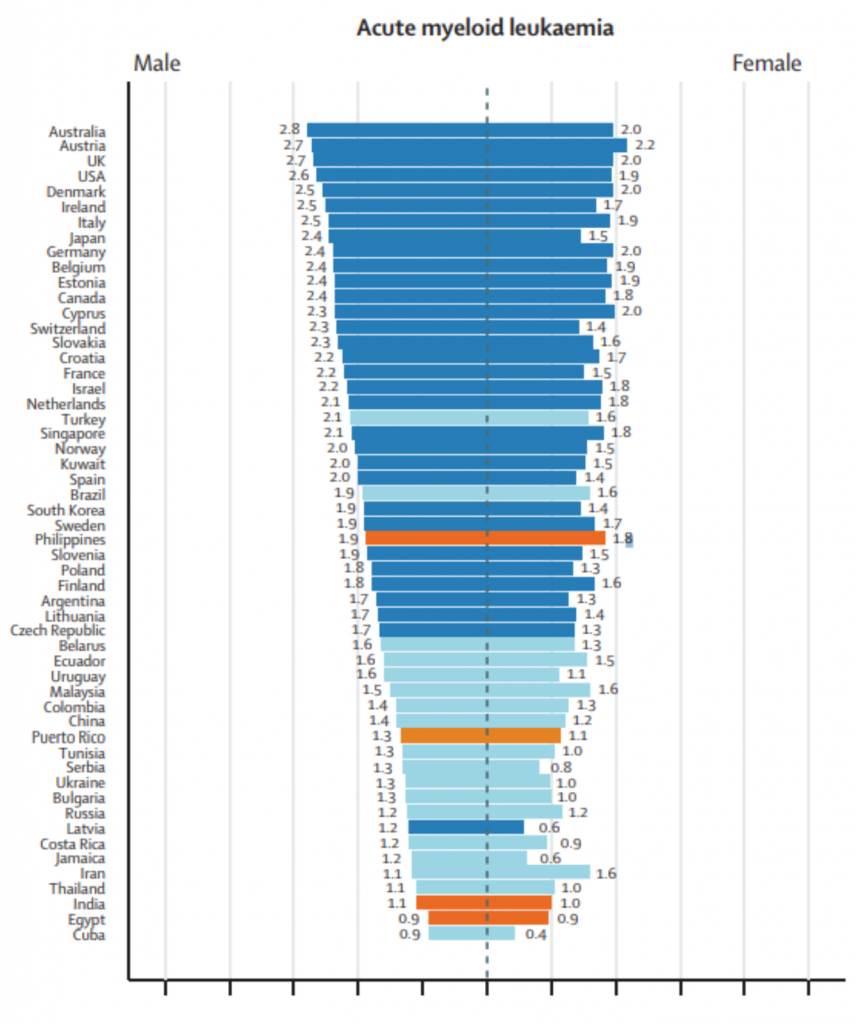
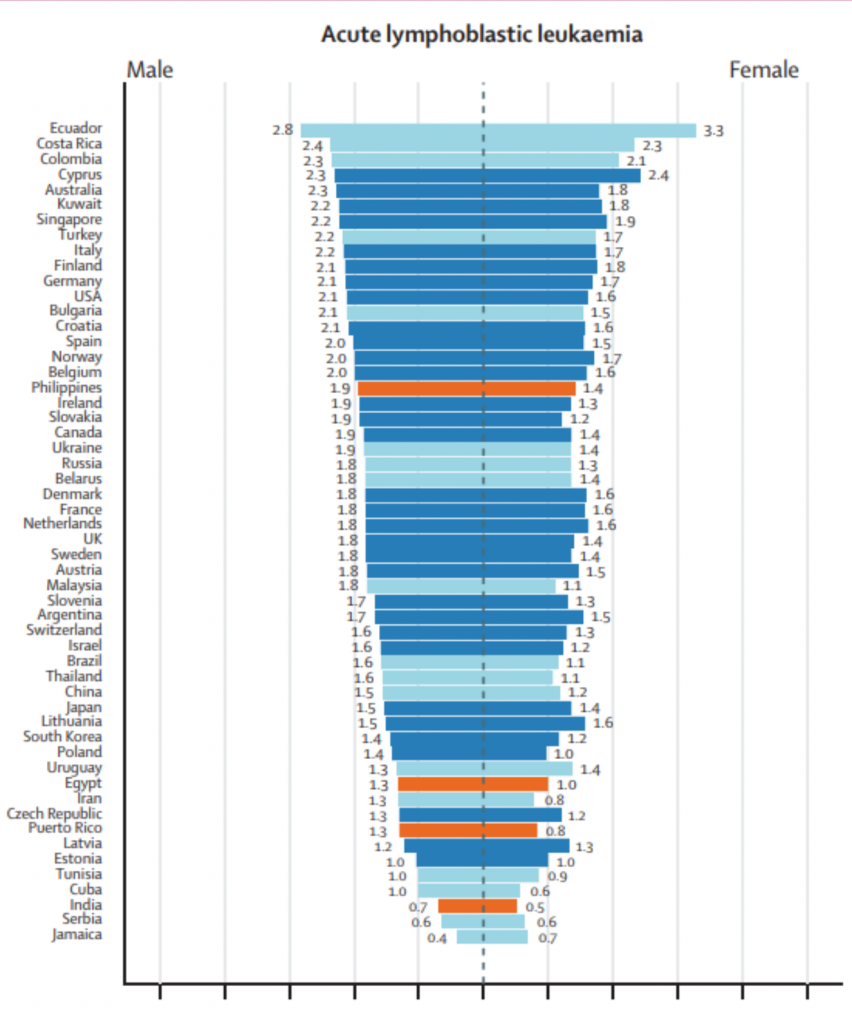
Per the 2018 Lancet Study, South America has some of the highest rates of acute lymphoblastic leukemia (ALL). The three countries with the highest age adjusted rates of incidence are Ecuador, Costa Rica, and Colombia.
40 USC § 599
At the appropriate time in the licensing process, we expect the National Institutes of Health (NIH) to obtain advice from the Attorney General (as is required under 40 USC § 599) to determine if the “disposal to a private interest would tend to create or maintain a situation inconsistent with antitrust law.”
The Bayh-Dole Act provides that “Nothing in this chapter shall be deemed to convey to any person immunity from civil or criminal liability, or to create any defenses to actions, under any antitrust law” [35 USC § 211 – Relationship to antitrust laws].
The Bayh-Dole Act sets out the areas where the Bayh-Dole Act “shall take precedence over any other Act which would require a disposition of rights in subject inventions” [35 USC § 210 – Precedence of chapter], and mentions 21 separate statutes, but does not include 40 USC § 599.
The process has lacked transparency
The Federal Register Notice for the license was 550 words. The name of the company (ElevateBio) set to receive the license is mentioned just once. We searched the Internet and could find no web page for ElevateBio. The NIH refused to provide answers to several basic questions about the license (see exchange here). For example, when asked to identify the key staff, board members or investors of a company with no web page and no Securities and Exchange Commission (SEC) filings, the NIH answered:
“This information is generally publicly available. Any information provided to us that is not publicly available regarding personnel for ElevateBio is considered business confidential information”
The NIH refused to provide information on the federal investments in research and development (R&D) related to the patented invention, explain what measures if any would be used to address access in low and middle income countries, and if the patent term will be for the life of the patent or something shorter.
We have been asking the NIH for data on the costs of the chimeric antigen receptor T-cell (CAR T) trials the NIH has undertaken or funded, and the NIH has resisted providing useful information on that topic.
The key figures in ElevateBio have a record of aggressively pricing drugs
We were able to independently gather some information about the company. According to the Massachusetts business entity registry, ElevateBio is an LLC, first organized in November 2017 in Delaware, and registered in Massachusetts on June 15, 2018. The Massachusetts filling listed four managers: Ansbert Gadicke, David Hallal, Morana Jovan-Embiricos, and Vikas Sinha. Below are a few notes on each of the four managers from the Massachusetts filings.
Ansbert Gadicke
Dr. Ansbert Gadicke co-founded MPM Capital, a healthcare investment firm that invests in companies that develop new therapeutics, particularly for oncology. He was the lead investor and served on the board of BioMarin Pharmaceuticals, Idenix Pharmaceuticals (acquired by Merck & Co.), Radius Health and Mitobridge (acquired by Astellas), and most notably, Pharmasset, which developed the hepatitis C virus (HCV) treatment Sovaldi before being bought by Gilead Sciences for $11.2 billion.
David Hallal
David Hallal is an executive partner at Dr. Gadicke’s MPM Capital. Prior to joining the investment firm, he was CEO of Alexion Pharmaceuticals which developed Soliris, one of the most expensive drugs on the market. Soliris is classified as an orphan drug, and treats paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). As of May 2017, it was typically priced from $500,000 to $700,000 per year.
Morana Jovan-Embiricos
Dr. Morana Jovan-Embiricos previously served as as a Partner at MPM Capital, before founding F2 Ventures and F3 Ventures, biotech venture capital funds. She has led and managed a series of biotech investment funds in the US and in Europe. Dr. Jovan-Embiricos, along with Dr. Gadicke, serve as a members of the Board of Directors of TCR2 Therapeutics, an immuno-oncology company that focuses on T cell receptor-based cellular therapies.
Vikas Sinha
Vikas Sinha is also an executive partner at MPM Capital, and previously worked alongside David Hallal, as CFO at Alexion Pharmaceuticals. He has also served in many roles at Bayer, including as Vice President and CFO of Bayer Pharmaceuticals in the US, and Vice President and CFO of Bayer Yakuhin in Japan.
Considering the background of ElevateBio’s founders, the NIH can expect the price for the new CAR T treatment to be aggressive, unless the NIH includes provisions in the license to restrain the prices.
Low- and middle-income countries
We ask that the NIH limit the exclusivity in the license to countries that have per capita incomes that are at least 30 percent of the United States, and to provide a public and transparent set of commitments to ensure that the therapy is available broadly in countries that are low- and middle-income.
We support this request with the following comment.
According to the “United States Public Health Service Technology Transfer Policy Manual, Chapter No. 300, PHS Licensing Policy:”
“PHS seeks to promote commercial development of inventions in a way that provides broad accessibility for developing countries.”
Designated Countries
The NIH should further clarify the geographic area of the license. We note that according to the WIPO PatentScope web page, the designated countries for PCT/US2017/034691 (the International Patent Application listed in the notice) are as follows:
| Designated States: | AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW
African Regional Intellectual Property Organization (ARIPO) (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW) Eurasian Patent Office (AM, AZ, BY, KG, KZ, RU, TJ, TM) European Patent Office (EPO) (AL, AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, TR) African Intellectual Property Organization (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, GW, KM, ML, MR, NE, SN, TD, TG) |
This includes most of sub-Saharan Africa as well as many countries of middle- and low-income status in other regions. If the policy of the NIH is to withhold patents or exclusive rights in countries with low and middle incomes, it needs to address that in the license itself, and not permit patent filings in all of the currently PCT-designated countries.
MPP
We ask the NIH to reach out to the Medicines Patent Pool (MPP), in order to enter into an agreement that gives the MPP an option to negotiate non-exclusive open licenses for the inventions in developing countries. We note the MPP has recently expanded its mandate to treatments for cancer and other disease.
Definitions of countries with access challenges
We also ask the PHS to reconsider the use of the term “developing countries,” which is no longer the most useful way to describe a category of countries for which access is a challenge.
There is no consensus on how to define “developing countries.” The WTO allows its members to self-identify as “developing.”
Policy makers often prefer to use the term “low- and middle-income countries” (LMIC), but this also requires a thoughtful definition.
The World Bank publishes and updates a list of country classifications every year, but the World Bank definition is anchored in a methodology from the 1980s that was based in part upon the cost of buying food, a poor proxy for global wellbeing today.
The World Bank definition of “high income” was adopted in 1989 by the Bank’s Executive Directors on the basis of a staff report on per capita income measures. The high income threshold was determined by an “explicit benchmark of $6,000 per capita in 1987 prices,” and updated annually with an adjustment for inflation. With real growth in per capita incomes, the number of countries that qualify as high income has continued to rise, and at some point, most countries will probably qualify.
Our recommendation is that the NIH consider relative per capita income as a useful starting metric for policies designed to mitigate inequality of access, recognizing that in some cases other factors such as prevalence of a disease may be appropriate to consider. As noted above, 30 percent of U.S. per capita income is a good starting point for identifying countries with significant challenges in regard to access.
35 USC § 209
Assuming the NIH has conducted a proper analysis to determine if any exclusive rights are necessary to induce investments in R&D to bring the inventions to practical application, we ask the NIH to limit the “proposed scope of exclusivity” so that it is “not greater than reasonably necessary to provide the incentive for bringing the invention to practical application,” as is required by 35 USC § 209.
Such an analysis should include an estimate of the expected costs (adjusted for risks and the costs of capital) to bring the invention to practical application, as well as reasonable estimates of the revenue from the sale of the technology that would be necessary as an adequate incentive for that investment. If the expected investments are small (which seems to be the case given the modest size of the clinical trials for other CAR T therapies) then the NIH should limit either (1) the number of years of exclusivity, (2) the prices that can be charged, (3) the maximum revenue earned before exclusivity is reduced or eliminated, or (4) some combination of 1-3.
35 USC § 201(f) – definition of practical application
The Bayh-Dole Act defines certain terms in 35 USC § 201, including the term “practical application.”
(f) The term “practical application” means to manufacture in the case of a composition or product, to practice in the case of a process or method, or to operate in the case of a machine or system; and, in each case, under such conditions as to establish that the invention is being utilized and that its benefits are to the extent permitted by law or Government regulations available to the public on reasonable terms.[emphasis added
“Available to the public” and “reasonable terms” taken together include the price to the public being reasonable. For the public, the price is the primary term of the transaction.
Proposals for safeguards to protect the public’s rights in the patented inventions
We propose the following measures to protect the public’s interest in any license to ElevateBio.
No discrimination against U.S. residents in pricing
We ask that the NIH include language in the proposed exclusive license to ensure that the prices in the U.S. for any health technology using the inventions are not higher than the median price charged in the seven countries with the largest gross domestic product (GDP), that also have a per capita income of at least 50 percent of the United States, as measured by the World Bank Atlas Method.
We consider this a modest and indeed minimalist request to protect U.S. residents, who paid for the R&D that created the licensed inventions.
We further note that President Donald Trump and Secretary Alex Azar have made recent comments in support of policies to impose international reference pricing for certain Medicare Part B drugs in some geographic areas, justified in part by the goal of reducing discrimination against U.S. residents. In this case, when the R&D for a treatment is subsidized by U.S. taxpayers, the rationale for including a non-discrimination provision is even stronger.
Additional provisions on affordability
The NIH should require that prices for products in the United States that use NIH-owned patented inventions do not exceed the estimated value of the treatment, as determined by independent health technology assessments selected by HHS.
The NIH should also create an obligation to set prices low enough that patient co-payments under third party Medicare programs are affordable.
Reduce term of exclusivity when revenues are large
In addition to an external reference pricing test, we propose that the exclusivity of the license in the U.S. should be reduced when the global cumulative sales from products or services using the inventions exceed certain benchmarks.
Given the modest cost of acquiring an NIH-patented invention, the amount of money the developer needs in sales to justify additional investments in R&D is reduced, as compared to cases where a company develops or acquires the technology from non government sources.
This request is consistent with the statutory requirements of 35 USC § 209, which demands that “the proposed scope of exclusivity is not greater than reasonably necessary to provide the incentive for bringing the invention to practical application.”
One possible implementation of revenue benchmarks is as follows: exclusivity will be reduced by one year for every $500 million in revenue equivalents, earned after the first $1 billion, where revenue equivalent is defined as global cumulative sales plus market entry rewards as well as government grants or tax credits, for the product or products using the invention. However, the NIH could choose different benchmarks, including even lower benchmarks, if the data on R&D costs will support a lower threshold, so long as the limits on exclusivity address the requirements of 35 USC § 209, in that the incentive is “not greater than reasonably necessary.”
Test data
In addition, we ask the NIH to include provisions that would require the licensed patent holders to waive any exclusive rights regarding test data that may exist in any country with a per capita income less than 30 percent of U.S. per capita income. This is important because a number of trade agreements and bilateral pressures force low and middle income countries to enact laws granting exclusive rights in test data, in most cases, without the possibility of exceptions, even in cases involving excessive prices.
A provision waiving exclusive rights in test data in countries with lower incomes is necessary for the NIH to implement the PHS policy “to promote commercial development of inventions in a way that provides broad accessibility for developing countries.”
Transparency
The licensee should be required to file an annual report to the NIH on the research and development costs associated with the development of any product that uses the inventions, including reporting separately and individually the outlays on each clinical trial. We will note that this is not a request to see a company business plan or license application. We are asking that going forward the company be required to report on actual R&D outlays to develop the subject inventions.
Reporting on actual R&D outlays is important for determining if the NIH is meeting the requirements of 35 USC § 209, that “the proposed scope of exclusivity is not greater than reasonably necessary to provide the incentive for bringing the invention to practical application.”
Sincerely,
Organizations
Health GAP
Knowledge Ecology International (KEI)
Public Citizen
Social Security Works (SSW)
Union for Affordable Cancer Treatment (UACT)
Individuals
James Love
Ophira Ginsburg, MD
